Stem Cells Engage In Dialogue With The Cells That Regulate Their Futures
Dialogue, not a monologue, is the basis of all good communication. Stem cells are no exception. Recent University of Washington (UW) research has found an early indication of two-way cellular communication in the miniscule niches of the body where the futures of stem cells are determined.
Stem cells require these niches - nest-like microenvironments made up of regulatory cells - in order to self-renew. Stem cells can divide and turn into many types of new cells. The niches help regulate the amount and kinds of new cells produced to meet current demands.
The niches also help maintain a supply of stem cells for later use. Inside your body, for example, there are separate niches for stem cells that will become blood, for cells that will become skin, and so on. Niches are places where your stem cells can replenish themselves and your tissue cells throughout your lifetime.
Problems in the niches can lead to diseases in the body. For example, if cell multiplication in a niche gets out of hand, cancer might form. A decline in cell production might contribute to the frailty of old age.
While a few stem-cell niches have been known for a long time, what's been harder to discover are the characteristics of the cells making up these niches and how they make it possible for stem cells to do their job. Signaling between cells in the niche plays a role in stem-cell upkeep and development. Most research has focused on the signaling of niche cells to stem cells.
"We looked at the possibility that two-way communication exists between stem cells and niche cells," said UW stem-cell niche researcher Hannele Ruohola-Baker, professor of biochemistry and a member of the UW Institute for Stem Cell and Regenerative Medicine. "Demonstrating that stem cells can contribute to niche function has far-reaching consequences for stem-cell therapies and may provide insight on how cancer might spread throughout the body via populations of cancer stem cells."
Ruohola-Baker added that stem cells hold high hope in regenerative medicine: tapping into the ways cells repair the body to create therapies to fix or replace injured tissues. She mentioned that it is thought that most, if not all, adult tissues contain stem cells.
Through self-renewing division," she explained, "stem cells replenish the stem cell pool. They also produce progeny that change into other, specialized cells. Importantly, stem cells have the capacity to divide throughout the life of an organism. They do so through regulated external stimuli that may initiate from stem cells.
This regulation of cell division needs to be tightly controlled." Too little division results in poor maintenance of tissues, while too much can result in tumors or other malignancies.
Her lab used the germline stem cell niche, found in the ovaries of fruit flies, as a model system. The production of fruit fly eggs depends on the presence of a renewable source of stem cells in the ovary of the adult fruit fly.
Inside the fruit fly ovary are structures called germarium which contain tiny cradles made of cap cells that nurture stem cells. Each such cradle contains two to three stem cells preparing to become fly eggs that are cuddled in a niche composed of three to six cap cells. Cap cells adhere to stem cells and this close contact may allow cap cells to play critical roles in communicating with stem cells.
The research team looked at a kind of signaling that usually depends on direct contact between cells, called the Notch pathway. The Notch protein is like a trigger poking out of a cell that can activate a mechanism inside the cell. When this trigger is pulled by proteins, called Delta and Serrate, from another cell, proteins are freed inside the cell to travel to the cell nucleus and turn on various genes.
According to Ruohula-Baker, the Notch pathway plays an important role in many stem-cell niches, including those in the blood system, gut, breasts, and muscles. However, in many cases it hasn't been clear which cells send and which ones receive the signaling protein.
The UW researchers analyzed the role of the Notch signaling pathway in both the stem cells and the cap cells. They found that either an increased production of Delta protein in the stem cells, or the presence of activated Notch protein in niche cells, resulted in up to 10 times the normal number of niche cells. These extra niche cells in turn resulted in a larger population of stem cells.
On the other hand, when stem cells don't produce functional Delta protein, they cease to be stem cells and soon leave the niche. The researchers also found that the receiving end for the Notch pathway, the trigger, is required in the niche cells, making them receivers of signals, not just senders. Work by other scientists had shown that TCF-beta signaling from niche cells is required to maintain active stem cells.
"Our study now shows that stem cells use the Notch pathway to signal to neighboring cells to maintain an active niche, and in turn, the niche induces and maintains the fate of the stem cells," Ruohola-Baker noted. "This is a first indication of a dialogue taking place between the stem cells and the niche that supports them.
It is tempting to speculate that maybe multiple potential niches exist for stem cells in our bodies that can be turned on to action when signaling stem cells are in the neighborhood. It may very well be that the power of cancer cells to spread comes from this natural ability of stem cells to make a home when in a hospitable environment. We all need a home, and stem cells with their strong survival instinct are active homebuilders."
The study will appear in the Dec. 5 issue of Current Biology, but is already online at the journal Web site. The research was supported by grants from the American Heart Association, the National Institutes of Health, and the American Cancer Society.
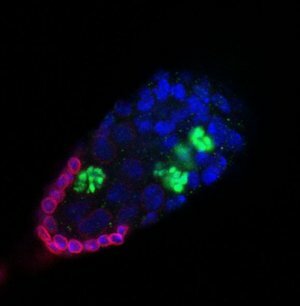
"Over-activation of a signaling pathway leads to an enlarged niche and over-production of stem cells. Red marks the niche cells, green marks a dividing stem cell, and all cell nuclei are marked blue. (Image courtesy of University of Washington)"
Source: University of Washington
|